

The Terrible Truth about Antacids
Reprinted from Nature’s Field
By David Satterlee
Many people routinely use antacids for relief of heartburn or as a calcium supplement. The truth is that both of these choices are usually nutritionally unsound.
The Bottom Line
Stomach acid is required for good digestion. Poor digestion produces heartburn. People with heartburn take antacids. Antacids reduce stomach acid.
OOPS.
Stomach acid is required for good calcium absorption.
Calcium carbonate antacids neutralize the stomach acid needed for their absorption. OOPS.
The stomach’s job is to produce and hold digestive acid and enzymes. When antacids are used regularly, the stomach senses this and, over time, increases its acid production rate setpoint. Taking antacids to reduce acid can lead to the production of excessive acid. OOPS.
Indigestion
Often, it is a LACK of stomach acid (PDA or Food Enzymes), not an excess that creates symptoms of indigestion. Naturopathic physicians have found that supplementary digestive acid and enzyme supplements can improve digestion and thus eliminate symptoms of indigestion.
Lack of stomach acid can also result in food allergies, nausea after taking supplements and rectal itching. It can be indicated by weak fingernails, anemia, chronic parasites, fungal infections, and acne.
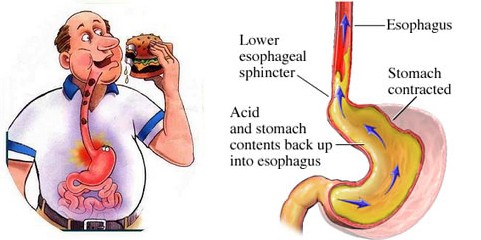
 Heartburn and Gastric Reflux
Heartburn and Gastric Reflux
Most digestive discomfort is a feeling of gaseous, bloated fullness. The pain feels like burning that radiates upward. Heartburn is most often the result of gastric juices refluxing up into the esophagus above the stomach. The discomfort of heartburn usually gets worse if you lie down because this makes it easier for gastric juices to back up.
What could make normal stomach digestive fluids flow up where they don’t belong? Usually overeating or factors such as obesity or pregnancy act to displace the contents of the stomach. Also smoking, alcohol, coffee, soft drinks, fried foods, etc., can weaken the sphincter muscle between the stomach and the esophagus. This muscle normally forms a one-way valve that prevents gastric reflux.
The stomach manufactures hydrochloric acid to break down proteins. But, as people age, they may produce less HCl, which can affect the amount of protein they can break down and ultimately absorb. Inefficient protein digestion can affect the viability of the intestinal flora that feast on these compounds.
|
|
|
The Problem of Gastric Ulcers
The stomach contains both hydrochloric acid and an enzyme called pepsin which are required for digestion of proteins. These are both normal and desirable but are also capable of digesting the stomach, which is made mostly of protein. The stomach normally produces a mucus coating that lines the inside to protect it from being digested. The best healing approach is to help the stomach to produce a healthy protective coating.
What do Antacids do?
Antacids reduce the acidity of the stomach by chemically absorbing or neutralizing some of the hydrochloric acid. Reducing stomach acid can temporarily relieve irritation of a weakened or exposed stomach lining. It can also reduce the acidity of stomach contents which become refluxed into the esophagus.
What About Antacids as Calcium Supplements to Avoid Osteoporosis?
Some antacids contain calcium carbonate and are advertised as calcium supplements. Calcium carbonate is an insoluble salt that must be ionized by stomach acid before it can be absorbed. About 40 percent of postmenopausal women (who are often concerned about osteoporosis) are severely deficient in stomach acid and can only absorb about 4% of the calcium in this form. Calcium carbonate greatly increases the risk of kidney stones, particularly when milk is also used regularly.
Here is one for your “haven’t-got-a-clue” file. Hippocrates magazine, May/June 1990 recommends: “Take antacid calcium supplements with meals, when there is enough acid in your stomach to aid absorption.” There are MUCH better forms of calcium. Although calcium citrate, for instance, is also an antacid, it is already in a form that the body can use and is absorbed much more effectively.
 Calcium Carbonate and Acid Rebound
Calcium Carbonate and Acid Rebound
Calcium carbonate is especially fast-acting. Within a few hours, however, the body will overcompensate by producing an extra surge of acid.
Problems with Sodium Bicarbonate
When used for long periods of time, sodium bicarbonate can cause increased acidity throughout the body. Highly acidic body chemistries (systemic alkalosis) are involved in health problems such as arthritis, kidney stones, nausea and mental confusion.
Aluminum Hydroxide and Bone Loss
Antacids containing aluminum can contribute to phosphate deficiency when used for long periods of time. This is because the phosphate in food reacts with the aluminum in the antacid to form a solid material that is simply passed in the stool. In order to keep enough phosphorus in the blood, the body will take it out of bones. Bone demineralization is especially a problem for the older people who tend to use these products regularly.
Aluminum and Impaired Mental Function
Although the FDA and manufacturers say that aluminum in antacids is not absorbed, studies since 1986 have shown that it is; especially in cases of kidney problems and in the presence of acidic foods such as citrus fruits or soda pop. There is increasing evidence that aluminum is involved in Alzheimer’s disease, Parkinson’s disease, Lou Gehrig’s disease and some other nervous system problems.
Magnesium in Antacids
Magnesium salts such as magnesium-oxide, magnesium-hydroxide and magnesium-carbonate are often used. Although they are milder antacids, they are also laxative and can cause diarrhea. They can be a problem for people with poor kidney function.
Other Side Effects of Antacids
Use of antacids can cause bowel irregularities including constipation, nausea and diarrhea with occasional vomiting. They can lead to kidney stones, demineralization of bones, bone pain and muscle weakness with cramping. They can be counted on to produce malabsorption of nutrients.
Diseases Related to Low Stomach Acid
When food, especially protein, is insufficiently digested into very small molecules, larger molecules can be absorbed by the body. These “foreign invaders” trigger the immune system and produce food allergies.
When a person doesn’t get full benefit from foods, even the best diet can be inadequate. You can’t assimilate nutrients until they have been adequately broken down by digestion. The list of diseases that have been associated with low gastric acidity include, but are not limited to:
Anemia, arthritis, asthma, autoimmune diseases, celiac disease, dermatitis, diabetes, eczema, gallbladder problems, hepatitis, lupus, osteoporosis, psoriasis and problems with over- and under-active thyroid glands.
This information is for educational purposes only. Consult with a qualified health practictioner for all serious or persistant illness. Copyright © 1999 by Robinson & Horne, L.C., P.O. Box 1028, Roosevelt, UT 84066. This material may be duplicated for educational purposes only (not for resale) provided it is not altered in any way.
Stimulate production of and/or supplement stomach acid and enzymes
By Steven Horne, RH (AHG) & Kimberly Balas, ND
There are two ways to increase stomach acid and enzymes. One is to take supplements and the other is to take herbs and nutrients that stimulate their production. With SIBO (Small intestinal bacterial overgrowth) it is normally necessary to do both.
To determine how much Betaine Hydrochloric Acid (HCI) you need, you can do a hydrochloric acid challenge test.
Note: do not perform this test if you have an active ulcer or a history of ulcers.
To do the test, take a capsule of PDA prior to a meal. If you notice no burning, increase to two capsules the next meal. Proceed until you notice a mild burning sensation, then immediately reduce your dose to the number of capsules that preceded the burning or heat sensation. Most people find a comfortable dose between 2-4 capsules.
If one or two capsules causes burning, you either don't have low stomach acid or your reflux is so severe that you won't be able to take HCI until you get it under control. Also, remember that the more protein you eat with a meal, the greater the need for HCI, so you can vary the dose with the size and content of your meals. Also, if you have severe digestive problems, you may also wish to take a complete food enzyme that has HCI and pancreatic enzymes.
Within 3-6 months most people feel a warmth in their stomach with the same dose they have been taking. When this happens it is time to decrease your dose and start weaning off of the PDA.
You can also mix one capsule of goldenseal powder with one teaspoon of Digestive Bitters and take this 15-20 minutes prior to meals with one to two glasses of water. A small pinch of a natural salt can also be taken at the same time, as this also helps stimulate HCI production by providing chloride.
Bitters are contraindicated if you have digestive atrophy. So, if you have dry mucus membranes, as evidenced by a dry and withered (or shriveled) looking tongue, don't take bitters because they dry the mucus membranes. Try Spleen Activator instead.
A lack of HCI may also be due to a lack of the following nutrients: chloride (low serum levels), zinc and thiamine. These are primary nutritional factors required for the synthesis of hydrochloric acid.
|