To Our New Site |
To Our New Site |

pH: Measuring & Balancing Simplified
By Jan Vandergriff and other authors
Our society is plagued with a torrent of health concerns. Many herbalists and nutrition now believe the explanation for this may come down to two small words: acid and alkaline.
AN IMPORTANT ISSUE
High acidity can affect all major body systems, especially the digestive, intestinal, circulatory, respiratory and immune systems. A pH-balanced environment maintains proper metabolic function and allows the body to function optimally. It also maintains alkaline reserves that are used to meet emergency demands.
UNDERSTANDING pH
pH is a measure of the acidity or alkalinity of a solution. The lower the pH the more acidic the solution is. The higher a pH number, the more alkaline the solution is.
YOUR BODY'S CHEMISTRY
Water is the most abundant compound, comprising 70% of the human body. The body has an acid-alkaline ratio called pH (potential of Hydrogen), which is a balance between positively charged ions (acid forming) and negatively charged ions (alkaline forming)
The body continually strives to balance pH. During times of imbalance, however, body systems can become weakened and these weaknesses may manifest themselves outwardly. Because our bodies naturally use hydrochloric acid to break down foods and nutrients, the optimal saliva and urine pH for our bodies is slightly acidic, around 6.4 - 6.5.
TEST YOUR ACIDITY OR ALKALINITY WITH OUR pH TEST PAPER ROLL
The Test paper |
Safe ranges |
| Caution: DO NOT apply the test paper on your tongue. For saliva readings, simply add some saliva on your finger tip and then apply it to a piece of test paper, wait a few seconds and check the color. | |
 |
|
The best time to test your pH is about one hour before a meal or two hours after a meal.
Test your pH two days a week.
The readings
MY URINE AND SALIVA pH RECORDS |
||||
TIME OF THE DAY |
DIET
[WHAT I ATE] |
Saliva
pH |
Urine
pH |
Energy Level (What I feel like ) |
| 1 | ||||
| 1 | ||||
| 1 | ||||
| 1 | ||||
| 1 | ||||
| 1 | ||||
| 1 | ||||
Write down the number (color) on your daily monitoring table above.
WHEN YOU WAKE UP
First thing in the morning, your eyes open up, you roll over and test your saliva pH.
In a best situation, your pH reads 6.4. (At night it should be a bit more alkaline up to 7.2 is fine)
Individuals with either chronic degenerative diseases or those setting themselves up for such will see their wake up saliva from 5.5 or lower with concurrent urine pH as low as 4.5.
These values represent a long term acid stress on the body.
Generally this means that an individuals alkaline reserves are very low to depleted.
In general you do not want to see a wake up saliva pH below 6.1.
Urine test numbers should be the same as saliva numbers (6.4 in the morning and up to 7.2 at night)
RESULTS OF URINE PH
It indicates how well your body is assimilating minerals, especially calcium, magnesium, sodium and potassium. These are called the "acid buffers" because they are used by the body to control acid levels.
When acid levels begin to increase, the body becomes less capable of excreting acid. It must either store the acid in body tissues, or buffer it - that is, borrow minerals from organs, bones, etc., in order to neutralize the extra acid.
SALIVA TEST
You sit down to eat, you get the aroma of your favorite mealtime dish, you are ready to eat that "healthy" food... and something begins to happen in your mouth. You begin to salivate.
This is a reflection of the enzyme amylase kicking in for the starch digestion process. This enzyme needs a range of pH ideally around 7.2 pH.
So if you have adequate alkaline reserves in your body, testing your saliva pH as you salivate before a meal should give you a pH reading of around 7.2.
If your pH is not getting up to at least 7.0, you can assume there is stress in your alkaline reserves and the further below 7 it goes, the more depleted are those reserves.
You could also suspect digestion all around is not doing so well. This typically indicates a longer term problem and more serious effort needs to be applied to help restore overall health.
IF YOUR BODY IS TOO ACIDIC
Most people who have unbalanced pH are "acidic." This condition forces the body to borrow calcium, sodium, potassium and magnesium - from vital organs and bones to buffer the acid and safely remove it from the body. This process can weaken these organs and bones over time.
|
IF YOUR BODY IS TOO ALKALINE
Though relatively uncommon, high alkalinity in the body causes many of the same kinds of mineral problems as acidity. It often takes longer for a person who is "alkaline" to achieve balance than one who is "acidic."
Alkalinity may lead to:
· Digestive system sluggishness,
· Intestinal system concerns, including poor elimination.
· Respiratory system compromise.
· Immune system concerns.
· Urinary system weakness.
· Nervous system exhaustion.
REMEMBER... Minerals are used to buffer acids.
THE BODY'S ACID MANAGEMENT
1. Excretion of acids
colon, kidneys, lungs, skin
2. Buffering of acids
calcium, magnesium, sodium, potassium
3. Storage of acids
tissue, joints, muscles, arteries
KEEPING THE BALANCE RIGHT FOR OPTIMUM HEALTH
Your body is able to assimilate minerals and nutrients only when its pH is properly balance.
When pH is OUT of balance, herbal programs can be negatively affected.
It is possible for you to be taking in healthy nutrients and yet be unable to absorb them. This is true because, while some minerals can be utilized through a wider range of pH than others, all provide their greatest benefits when the body's pH level of urine fluctuates between 6.0-6.4 in the morning and 6.4-7.0 in the evening, and the saliva stays between 6.4-6.8.
For example, though the body can utilize iron while at a pH anywhere between 6.0 and 7.0, it can only fully utilize iodine when body pH ties between 6.3 and 6.6. Thus, it is in your body's interest for you to maintain the best pH balance possible.
For extra support for an acidic condition, be sure to follow these steps to improve your digestion and resupply your minerals.
A PROPER pH IS A MUST FOR MUCH NEEDED "NUTRIENTS" TO BE ABSORBED BY THE BODY
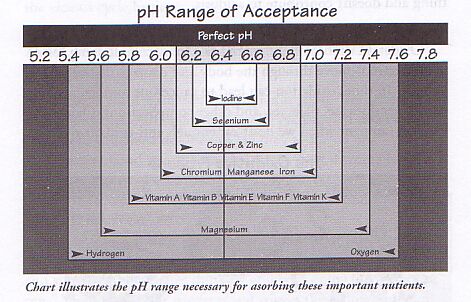 |
If one has a urine PH above 6.8 or below 6.4 some or many nutrients are not being absorbed.
(IODINE FOR EX. ... VERY IMPORTANT FOR THE THYROID, IS PROPERLY ABSORBED WHEN THE pH IS BETWEEN THE NARROW RANGE OF 6.3 - 6.6 )
This is because vitamins and minerals are affected by enzymes and digestive juices produced by the body. When the PH deviates too far, certain enzymes are not as active and, therefore, nutrients cannot be absorbed. Microorganisms such as yeast, bacteria and parasites can also change the environment of the body to make absorption more difficult.
Remember, you are what you eat, but you really are what you digest.
From Dr. Kasuhiko Asai, Ph.D.
Although I shall refer to the reason for ill health together with the conditions for treatment
of disease on many occasions. perhaps it would be useful to summarize the causes for an
oxygen deficiency in the body at this point since this seems to be the root of all body
disorders.
1. Though acidity has long been recognized as a problem, perhaps one reason why ill
health is so rampant is that most people do not bother to find an adequate explanation as to
why this condition is detrimental to health.
Although it may sound complicated, the scientific
answer is rather simple. An acid constitution means that the blood contains an excess of
positive hydrogen ions (H + ) which use up the oxygen in a living body by combining with it to
form an hydroxyl group. When an excess of (H + ) accumulates in the body, there is
insufficient oxygen to consume them. As a consequence the blood will acidify and an oxygen
deficiency will be created.
The problem of oxygen deficiency has also become a controversy
in recent years. An acid constitution should be averted by all means. An acid constitution
leads to an oxygen deficiency which results in various diseases, including cancer.
2. The intake of foods which contain an excess of unsaturated chemical compounds can
be noted as another major cause of oxygen deficiency. Unsaturated compounds have a
surplus of molecular "hands" which combine with oxygen in the body to produce oxonium
compounds, thereby depleting the body's supply of oxygen. The
chemical
nature of substance is to move from an unsaturated state at which they can establish their
existence. In this context, it is important for those who want to stay healthy to note that
natural foods contain the fewest unsaturated compounds, while these compounds are
conspicuously abundant in refined foods.
In my mind, carcinogenic or cancer producing
substances and unsaturated compounds with their excess of molecular "hands" to use up the
available oxygen in the
body are one and the same.
3. The other major cause of oxygen deficiency in the body, perhaps that to which we
should pay the most attention, is the mind. A theory of Professor Hans Selye of the
University of Montreal, Canada, is that not only humans but animals develop an unbalanced
secretion of hormones, particularly adrenal hormones, if subjected to prolonged stress. An
imbalance hormone secretion will also lead to acidification of the blood and thereby create a
condition for oxygen deficiency which ultimately results in disease.
With the recent remarkable progress in biochemistry, many warnings have been issued on
the relationship between health and diet. Moreover stress is fast becoming recognized as a
major cause of disease. Again, I wish to re-emphasize the necessity of maintaining a
constitution that will not produce an oxygen deficiency. This is a conclusion reached after
several years of laboratory work with the organic germanium compound and, to date, all the
facts have supported it without exception.
See List of Acidic/Alkaline Foods
|